Existing scientific models are constantly being revisited as analytical methods become more sophisticated. What has recently come under scrutiny is how molecules are organized on the surface of large volumes of salt water.
Researchers at the University of Cambridge in the UK and the Max Planck Institute for Polymer Research in Germany have discovered that charged particles, or ion, is not active at the very surface of the solution, as previously thought. Instead, they are located in layers below the surface.
This discovery requires a redrawing of the textbook model. A University of Cambridge press release explains:.
“Our study shows that the surface of a simple electrolyte solution has a different ion distribution than previously thought, and that the ion-rich subsurface determines the composition of the interface.” To tell Yair Littman, a theoretical chemist at the University of Cambridge.
To make the discovery, the team used an upgraded version of a laser illumination technique called . Vibration sum frequency generation (VSFG) measures molecular vibrations at the smallest scales with incredible precision.
Together with a model that leverages neural networks, this improved technique allows researchers to determine whether ions on a surface are positively charged (cation) or negatively charged (anion).
In addition to detecting the subsurface layer of ions, the new study also reveals that these ions can be oriented not only in one direction, but both up and down, referring to the actual physical arrangement of the molecules. Ta.
“At the top there are several layers of pure water, then an ion-rich layer, and finally a bulk salt solution.” To tell Litman.
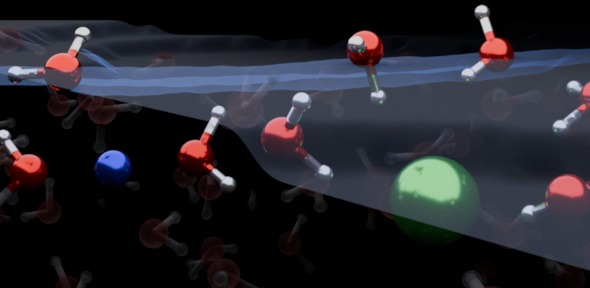
Simply put, this experiment reveals what happens at the boundaries of the simplest liquids. electrolyte solution. The arrangement of molecules tells how they react with their surroundings.
A thorough understanding of these layers and their arrangement can inform all kinds of other models, such as those for ocean surfaces, which are essential for predicting how climate change will affect the atmosphere. I can.
The researchers say their work could not only improve our understanding of the world around us, but also help develop all kinds of technologies that require combining solids and liquids, including batteries. suggests.
“These kinds of interfaces exist everywhere on Earth, so studying them can not only help our fundamental understanding, but also lead to better devices and technologies.” To tell Misha Bonn, a molecular physicist at the Max Planck Institute for Polymer Research, said:
“We are applying these same methods to study solid/liquid interfaces, which could have applications in batteries and energy storage.”
This study natural chemistry.

