artistic expression of Legionella pneumophila SidI-mediated effects on host cell translation. Credit: Image created by Advait Subramanian with creative assistance from Biorender.com.
The central dogma of molecular biology is that packets of information encoded within molecules of deoxyribonucleic acid (DNA) are first transcribed into molecules of messenger ribonucleic acid (mRNA), which are then translated/decoded to produce molecules called proteins. It is assumed that
Proteins are essential biological molecules made up of multiple small subunits called amino acids. These amino acids are linked together through peptide bonds and contribute to the final shape, size, and charge distribution of the protein as a sum of its amino acid moieties.
In order for cells to make proteins, they must decode the language of mRNA (nucleic acids) and translate it into the language of proteins (amino acids). This process is described interchangeably with mRNA translation or protein synthesis in molecular biology textbooks.
Inside cells, translation is carried out by molecular decoding centers called ribosomes. The ribosome itself is made up of dozens of proteins and RNA. In addition, many other regulatory factors are associated with ribosomes and help make the translation process fast, accurate, and tunable. In fact, if you count the number of proteins involved in this process, the number is over 100. That’s right, it takes over 100 proteins to make one. Therefore, the process of translation is highly energy and resource consuming, reflecting its great importance for the cell.
During translation, an important molecule that facilitates decoding is called tRNA (transfer RNA). tRNA is a “translator” that knows both the language of nucleic acids and the language of proteins. The tRNA brings amino acids (the building blocks of proteins) to the corresponding mRNA sequences (called codons), while the ribosome moves along the length of the mRNA, stringing the amino acids together at the same time. Thus, the ribosome-tRNA complex translates one language into another and performs important steps in protein synthesis.
As with any other process, cells do their best to maintain the efficiency and precision of protein synthesis. In fact, measurements in many different cells have shown that proteins are generally synthesized with high precision at an average rate of about 6 amino acids per second.
Pathogens target protein synthesis.
Many pathogens, including viruses, bacteria, and fungi, have evolved mechanisms that directly target the protein synthesis machinery within the cells they infect. This allows the pathogen to multiply, subvert the host cell’s defenses, and ultimately control host cell lysis, allowing it to release its progeny into further rounds of infection.
In these infected cells, the amino acid chains that make up proteins do not elongate at a uniform rate, and ribosomes pile up at specific positions on the mRNA being translated, slowing down the rate of protein synthesis in the host cell. . As an example, consider rush hour traffic on a freeway. Here, the car is the ribosome and the asphalt road is the length of the mRNA. Due to an untoward incident, cars start piling up on the highway, slowing down the traffic.
Previous evidence suggests that a pathogenic bacterium called Legionella pneumophila produces similar responses within infected cells: a slowing of the host cell’s rate of protein synthesis and stalling of ribosomes on mRNA, or the “protein synthesis highway.” This indicates that it will cause traffic congestion. Legionella pneumophila is an intracellular bacterium (infects and replicates inside cells) that causes Legionnaires’ disease, a debilitating atypical pneumonia.
We asked how and why Legionella pneumophila targets the steps of translation elongation.And we set out to answer these questions recent research Currently published natural cell biology.
The road to discovery is full of surprises
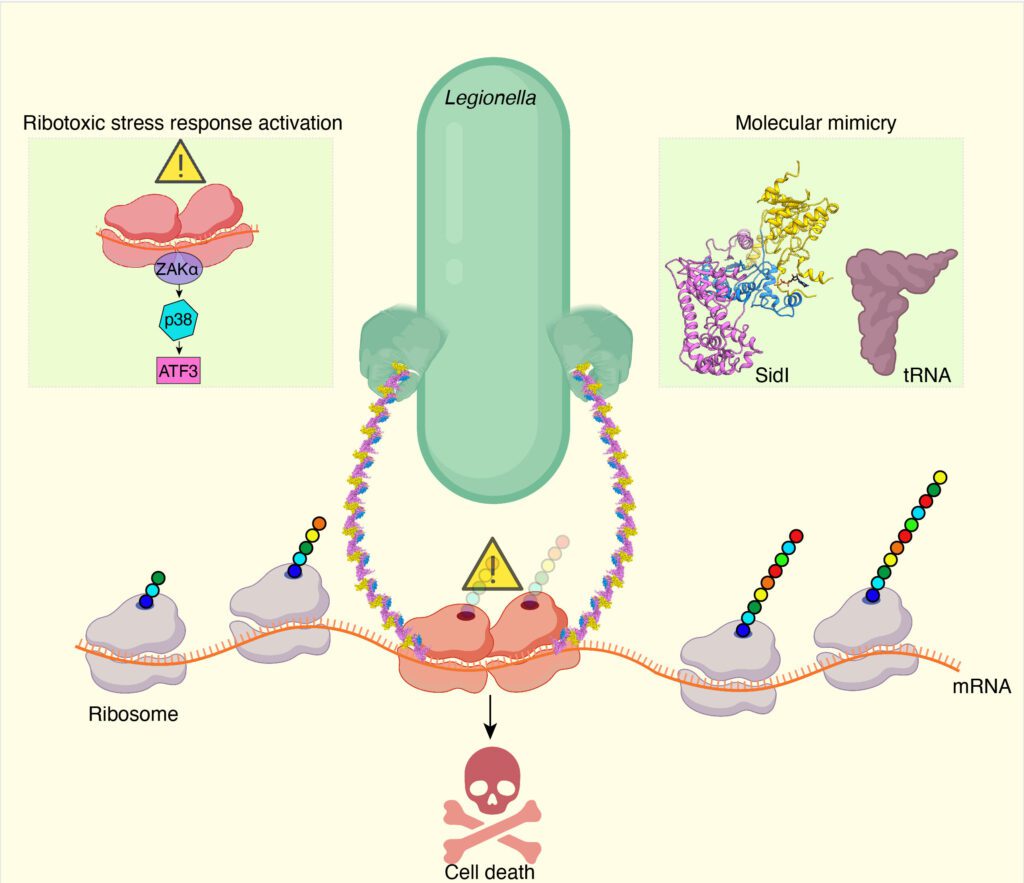
Legionella bacteria secrete several toxins into infected cells. Therefore, we first determined whether these toxins were equivalent in their ability to inhibit protein synthesis. This was our first surprise. Six of the toxins we tested had similar effects, but one Legionella toxin stood out from the rest of the pack, and it was called SidI (pronounced “Sid-eye”). Ta. We found that small amounts of SidI strongly inhibit protein synthesis. Our measurements showed that the potency of SidI is comparable to that of ricin, one of nature’s most potent toxins.
Our second surprise came when we solved the structure of this potent inhibitor by cryo-electron microscopy. This technique preserves the three-dimensional structure of biomolecules in solution by embedding them in a glassy ice environment (approximately -320°F). . Our structure revealed an interesting molecular mimicry.
Half of SidI has a structural fold found in enzymes called glycosyltransferases, while the other half resembles the shape and size of a tRNA molecule. Recall that tRNA carries individual amino acids to the ribosome and helps decode the mRNA. SidI pretends to be tRNA and tricks the ribosome into accepting it, but instead of bringing the amino acid to the ribosome, it stops the ribosome from translating.
We performed a series of experiments to confirm that SidI directly targets and modifies host cell ribosomes. This creates a condition in which the modified ribosomes on the mRNA move much more slowly than the following ribosomes, causing ribosome collisions. Using a highway analogy, this is similar to a car crash that occurs when the car in front of you has a problem and suddenly slows down.
To our knowledge, this combination of form (tRNA mimicry) and function (enzymatic activity) makes SidI a unique and unprecedented molecule in nature.
“Then what? [the] What are the consequences of such aberrant ribosome collisions in infected cells? ” we asked.
In trying to answer this question, we encountered a third surprise. We show that these colliding ribosomes are sensed by host cells and activate ribotoxic stress response pathways, ultimately leading to the accumulation of a master regulator of gene expression called activating transcription factor 3 (ATF3). I discovered that.
Remarkably, even though most protein synthesis is inhibited, the ATF3 protein bypasses this mRNA translation block and is induced at high levels. ATF3 enters the cell nucleus and coordinates the program that controls cell lysis. We believe that this mechanism may be important for escaping replicated bacteria into the extracellular environment and promoting further infection.
Outlook
Toxins from pathogenic microorganisms have long been used as precise molecular instruments to investigate fundamental processes occurring within cells. SidI joins this arsenal of natural tools. Fundamental insights from our study shed new light on the mechanisms by which pathogens use molecular mimicry to hijack critical processes to monitor optimal host cell function.
Interestingly, through elucidation of the molecular mechanism of SidI, we successively discovered important signaling nodes in the stress response pathway that are activated downstream of colliding ribosome stress. As our mentor, Professor Shaeri Mukherjee of the University of California, San Francisco, always says, “Bacteria are the best cell biologists!”
This story is part of science x dialogueresearchers can report findings from published research papers. Please visit this page Learn more about ScienceX Dialog and how to participate.
For more information:
Advait Subramanian et al, Legionella toxins exhibit tRNA mimicry and glycosyltransferase activity, target the translational machinery, and trigger ribotoxic stress responses. natural cell biology (2023). DOI: 10.1038/s41556-023-01248-z
Advait Subramanian is a cell biologist at Altos Labs Inc. in Redwood City, California. Previously, Subramanian was a postdoctoral fellow supervised by Shaeri Mukherjee and Peter Walter at the University of California, San Francisco, where the research described in this article was conducted. Contact email: asubramanian@altoslabs.com
Lan Wang is a structural biologist and assistant professor at the Hong Kong University of Science and Technology, Hong Kong. Previously, Lan was a postdoctoral fellow at the University of California, San Francisco, supervised by Peter Walter. Contact email: lanwang@ust.hk
Quote: How Pathogenic Bacteria Use Molecular Mimicry to Compromise the Cell’s Protein-Building Factory (December 5, 2023), https://phys.org/news/2023-12-pathogenic-bacterium-molecular-mimicry Retrieved December 5, 2023 from -compromise. html
This document is subject to copyright. No part may be reproduced without written permission, except in fair dealing for personal study or research purposes. Content is provided for informational purposes only.