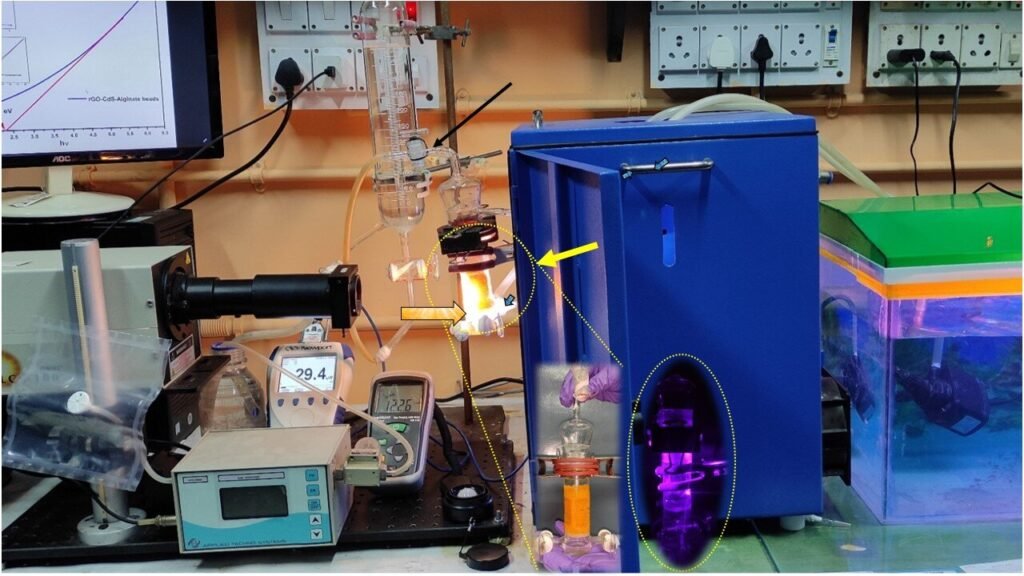
Actual photos of the continuous photocatalytic hydrogen production reactor and accessories setup.Credit: Elsevier International Hydrogen Energy Journal
× close
Actual photos of the continuous photocatalytic hydrogen production reactor and accessories setup.Credit: Elsevier International Hydrogen Energy Journal
Environmental pollution and affordable clean energy are two major sustainable development goals set by the United Nations General Assembly in 2015. All countries have set targets for decarbonization by 2050 and increasing the use of green hydrogen to reduce annual electricity consumption. .
Industry and research groups have collaborated to increase green hydrogen production and reduce production costs. In 2023, we find ourselves in the midst of a global energy crisis in major parts of Europe during the war, rising prices and shortages of liquefied natural gas, and worsening climate change.
Generally, green hydrogen is produced by water splitting in an electrolytic cell and photocatalyst. There are several barriers to commercial production of green hydrogen, such as high production costs, photocatalyst stability, catalyst performance, and seawater use.
Photocatalytic solar water splitting has opened new opportunities to produce low-cost green hydrogen in accordance with environmental protection. Sunlight is abundant in the environment, and the correct selection of high-performance and long-term stable photocatalysts could improve production and lower the price of green hydrogen.
In particular, the photocatalysts available for hydrogen production by water splitting are all in the form of powdered nanoparticles, which leads to metal loss and aggressiveness, resulting in reduced photocatalytic activity and impacting operating costs. Additionally, powder nanoparticle photocatalytic systems only operate in batch mode and cannot control the hydrogen production rate.
Alginate-based hydrogel photocatalyst and its trapped water Credit: Elsevier, International Hydrogen Energy Journal
× close
Alginate-based hydrogel photocatalyst and its trapped water Credit: Elsevier, International Hydrogen Energy Journal
Powdered nanoparticle photocatalysts contain semiconductors and can leach into water bodies and harm the ecological pyramid. Metal-organic frameworks supporting nanoparticles of alloys have been proposed to prevent metal agglomeration during reactions and enhance catalytic activity.
A team led by Professor Kajali Kargupta of the Nanoengineering and Sustainable Energy Laboratory in the School of Chemical Engineering at Jadavpur University in India has developed an eco-friendly and recyclable 3D organic alginate hydrogel encapsulated in bead-shaped photocatalysts. Research is published inside International Hydrogen Energy Journal.
These types of 3D metal-organic framework-based hydrogel photocatalysts can supply continuous hydrogen at a stable rate. The toxic effects of the semiconductor are minimized by encapsulating it in sodium alginate, a food-grade material.
Sodium alginate is the biopolymer of choice for photocatalyst-encapsulated millispheres. It is commercially produced from wakame extract. Over time, several research groups have formed various metal-polymer composites by immobilization of metal ions during the gelation process.
A pressure-driven flow-through system operating in both batch and continuous modes under full-band solar irradiation uses a novel 3D millisphere of organic alginate hydrogel-encapsulated photocatalysts with high water-holding capacity to Researched to enhance solar hydrogen production. The main focus was predicted on the role of enhanced adsorption of water molecules at the active sites of photocatalysts on the performance of solar-induced hydrogen production.
From a functional point of view, the addition of sodium alginate improves the photocatalytic activity and water retention capacity, enabling a continuous hydrogen production process. From an operational point of view, the presence of alginate increases the photocatalytic activity and water retention capacity, enabling a continuous hydrogen production process.
All spherical beaded alginate encapsulated photocatalysts function as miniature hydrogen generators or photocatalytic reactors. The alginate hydrogel also showed excellent recyclability and reusability. The reproducibility and linear scalability of the synthesis is confirmed by the fact that the total amount of hydrogen produced increases linearly with the number of photocatalyst-encapsulated beads, while the volume normalization rate remains constant.
The degree of hydration (both pre- and dynamic water adsorption) has a significant impact on the rate of hydrogen production. A flow reactor is used to produce hydrogen at a constant rate. When the inlet flow rate falls below a critical value, the production rate remains constant, indicating that each spherical catalyst acts as a miniature hydrogen generator.
Professor Kargupta has experience in translating laboratory-scale prototypes into practical commercial applications, and our multidisciplinary team has expertise in solar hydrogen production, fuel cell electrolyte membrane/electrode fabrication, and carbon sequestration. It contains. The research team is looking to expand the capacity of hydrogen produced to power portable fuel cells in remote locations.
The main chemical used for photocatalyst encapsulation is sodium alginate, which is considered a food-grade material (emulsifier, stabilizer, thickener, gelling agent) by the US Food and Drug Administration and the European Commission. Masu. Alginate hydrogel-based photocatalysts with suitable photoreactors will be assembled as high-storage batteries and fuel cells in the next two years. We plan to work with industry partners to scale up this high-performance photocatalyst on an industrial scale.
This story is part of science x dialogueresearchers can report findings from published research papers. Please visit this page Learn more about ScienceX Dialog and how to participate.
For more information:
Sayantanu Mandal et al., Superior photocatalytic solar hydrogen production with rGO-CdS millispheres encapsulated in organic alginate; International Hydrogen Energy Journal (2023). DOI: 10.1016/j.ijhydene.2023.09.137
Professor Kajali Kargupta of the Department of Chemical Engineering, Jadavpur University, received her Ph.D. He was from IIT Kanpur in 1998 on “Instability and Pattern Formation in Thin Films: The Role of Inhomogeneity, Evaporation and Slippage”. She has expertise in thin film systems, pattern generation, and the formation of various forms of nanostructures and their applications. She has successfully completed several projects sponsored by SERB DST, UGC, DBT, DRDO and has over 100 peer-reviewed journal publications. She has experience in the formulation of various forms of graphene-based bimetallic nanohybrid materials and their application as catalysts and electrocatalysts for hydrogen production. As part of her previous DST-sponsored project, she investigated the synthesis and characterization of graphene-based bimetallic nanohybrid catalysts for hydrogen production from sodium borohydride and borohydride electrooxidation. Based on composition-morphology-performance mapping, a novel rGO-based bonded core-shell G-Co-Pt nanohybrid catalyst exhibiting excellent electron transport properties is investigated as an ORR catalyst for hydrogen production and reducing Pt loading. Masu. Dr. Kargupta has experience in the synthesis and characterization of electrocatalysts for electrooxidation, oxygen reduction reactions, and fuel cell applications. She has studied photocatalytic and photoelectrocatalytic solar hydrogen production through water splitting. Her goal is to address key process bottlenecks and increase the efficiency of solar power to hydrogen conversion. Based on her experiments and quantum simulations, the roles of nanohybrid catalysts/photocatalysts and photoelectrocatalysts are analyzed and explored. Previously, as part of a major project at UGC, Professor Kargupta has researched various inorganic-organic nanocomposite membrane electrolytes and gel-type portable and durable proton-conducting electrolytes, especially for portable applications in fuel cells. Did. Professor Kargupta has worked on 10 sponsored projects as a PI and her Co-PI. She has also worked with NMRL, DRDO on mission projects related to fuel cell applications as a research service provider.
Sayantanu Mandal is currently pursuing his Ph.D. He completed his master’s degree from the Department of Chemical Engineering, Jadavpur University under the supervision of Professor Kajali Kargupta. For the past three years he has been working on hydrogen production and the fabrication of high temperature fuel cell electrolyte membranes. Currently, he is also serving as his PI for the Indian Science, Technology and Engineering Facilities Map (I-STEM) based project of the Government of India and his guide is Professor Kajari Kargupta (I-STEM/Catalyticgrant/acad_24/2022- 23) is also in charge. He is also a standing member of prestigious scientific world organizations such as the International Association of Engineers (IAENG) and the International Academy of Science and Engineering for Development (IASED) in Hong Kong. He also serves as a reviewer on the technical committees of MEAMT 2023, NanoMT 2023, and ICFMCE 2023.