The big advantage of nuclear fusion is that it can produce huge amounts of energy from just a few grams of fuel. More specifically, one gram of fuel in the fusion process can produce the equivalent of eight tons of oil. Similarly, the Sun can provide energy to the entire Earth through nuclear fusion reactions.
A similar thing happens with nuclear fission, the process currently used to generate energy when operating nuclear power plants. The amount of fuel (in this case uranium) required is very small compared to that required by thermal power plants that burn coal, gas, or oil. No matter how large-scale the use of fusion energy becomes in the future, the amount used (by extracting the necessary materials and releasing gases) will never be large enough to change the composition of the atmosphere. Furthermore, fusion is an energy production process that does not emit greenhouse gases.
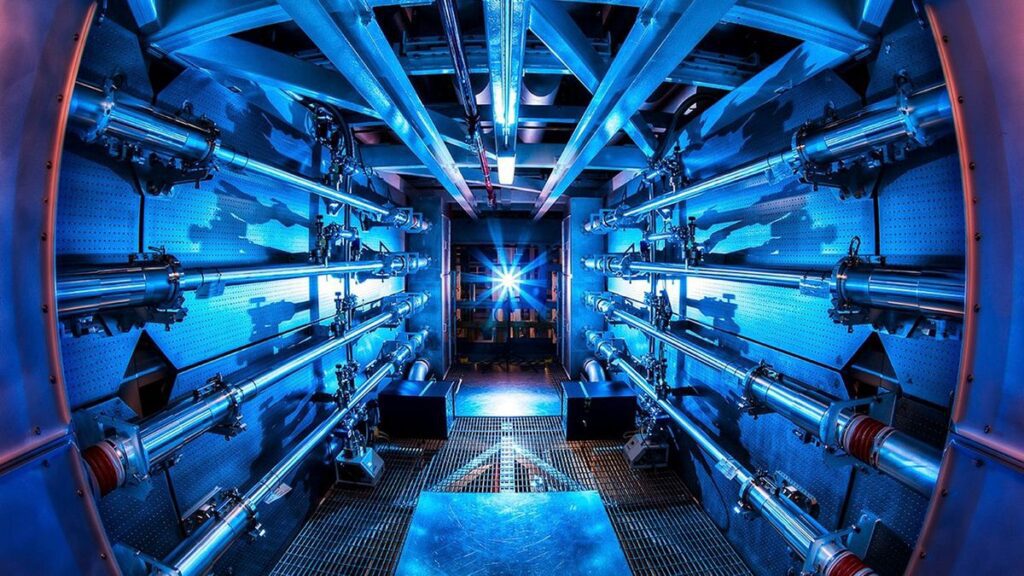
The widespread use of nuclear fusion reactors will require the use of hydrogen isotopes. Isotopes are atoms of the same element (same number of electrons and protons) but different numbers of neutrons. This feature means that different isotopes of an element have the same chemical properties but different physical properties. The lowest temperature fusion reaction is the reaction between deuterium and tritium. Deuterium is very abundant in seawater and can be extracted by hydrolysis. Tritium is then produced in the fusion reactor itself, since neutrons from the fusion reaction affect the regenerating mantle, which consists of lithium, among other elements. The neutrons and lithium atoms produce tritium as a byproduct, which is reused as fuel within the plasma. Plasma is a substance that produces energy by fusing atomic nuclei. It is an ionized gas with a temperature of over 11,000 degrees Fahrenheit.
Nuclear reactions occur not only by fission, but also by fusion, but they are different from the processes we know from the combustion of fuel, that is, processes based on chemical reactions. For nuclear fusion, the nuclei must be brought close together so that the nuclear force acts and strongly attracts each other. When they fuse, a new element is formed whose mass is less than the sum of the masses of the initial nuclei. This difference in mass (though hardly significant) has the ability to be converted into energy by Einstein’s famous equation E=mc². Please note that this process involves very light elements. Nuclear fusion uses isotopes of hydrogen, the lightest element in nature. Hydrogen is the first element on the periodic table because it has only one proton and one electron. And then there’s helium. When two hydrogen atoms combine in a nuclear fusion reaction, helium and extra neutrons are obtained, and these neutrons have a lot of energy.
To find out how much material is needed as fuel, it has a density that is one millionth of the density of the air we breathe in the plasma confinement devices used in nuclear fusion experiments. This means that there are very few particles. Because the density is so low, no matter how many reactions occur due to the release of helium, it cannot change the composition of the atmosphere. These emissions do not include CO₂, so they are not due to hydrogen consumption or emissions produced by fusion reactions.
sign up Get more English news coverage from EL PAÍS USA Edition with our weekly newsletter.