Graphical abstraction. credit: ACS nano (2024). DOI: 10.1021/acsnano.3c07853
For the first time, scientists have successfully trapped atoms of the rare gas krypton (Kr) inside carbon nanotubes to form a one-dimensional gas.
Scientists at the University of Nottingham’s School of Chemistry are using advanced transmission electron microscopy (TEM) techniques to bond Kr atoms one by one in “nano test tubes” that are less than 500,000 times wider in diameter. I captured the moment. of human hair. The research is published in ACS nano.
Scientists have studied the behavior of atoms ever since it was hypothesized that they are the fundamental units of the universe. The movement of atoms has a profound effect on fundamental phenomena such as temperature, pressure, fluid flow, and chemical reactions.
Traditional spectroscopy analyzes the motion of large groups of atoms and can use averaged data to describe phenomena at the atomic scale. However, these methods do not tell us what individual atoms are doing at any given time.
The challenge researchers face when imaging atoms is that they are very small, 0.1 to 0.4 nanometers, and can move at very high speeds in the gas phase, about 400 m/s, about the speed of sound. This makes direct imaging of atoms in motion extremely difficult, and continuous visual representation of atoms in real time remains one of the most important scientific challenges.
Professor Andrei Klobistov, from the University of Nottingham’s School of Chemistry, said: “Carbon nanotubes allow us to trap atoms and precisely position and study them in real time at the single-atom level. ). “In this study, we used ) atoms. Kr has a high atomic number, so it is easier to observe in TEM than lighter elements. This allows us to move the position of Kr atoms. We were able to track it as a dot.”
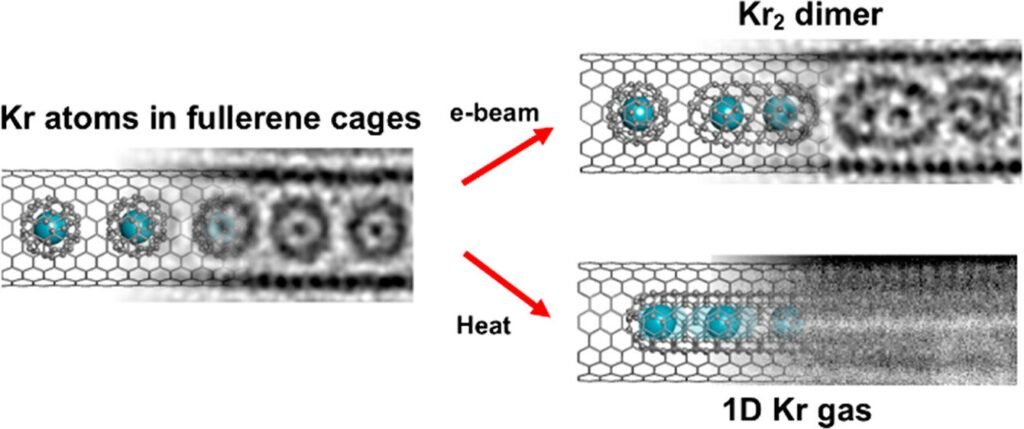
“To observe the process, we used the state-of-the-art SALVE TEM, which corrects for chromatic and spherical aberrations,” added Prof. Ut Kaiser, former head and senior professor of the materials science electron microscopy group at Ulm University. .Krypton atoms combine to form Kr2 pair. ”
“These pairs are held together by van der Waals interactions, a mysterious force that governs the world of molecules and atoms. This is because we can see the van der Waals distance between two atoms in real space. , an exciting innovation.” This is an important advance in the fields of chemistry and physics, and will help us better understand how atoms and molecules work. ”
The researchers used Buckminsterfullerene, a football-shaped molecule made of 60 carbon atoms, to transport individual Kr atoms into nanotubes. The accuracy of the experiment improved when Buckminsterfullerene molecules coalesced to form nested carbon nanotubes.
Dr. Ian Cardillo-Zallo, a student at the University of Nottingham who was responsible for the preparation and analysis of these materials, said: “Krypton atoms can be released from the fullerene cavity by fusing the carbon cage. This can be accomplished by heating with a “electron beam” or by irradiating it with light. Both the interatomic bonds between Kr atoms and their dynamic gaseous behavior can be studied by him in one of his TEM experiments. ”
The group was able to directly observe Kr atoms exiting the fullerene cage and forming a one-dimensional gas. Once the Kr atom is released from the carrier molecule, it can only move in one dimension along the nanotube channel due to the very narrow space. Atoms in a chain of bound Kr atoms cannot pass each other and are forced to slow down, like cars in traffic.
The researchers captured a key stage when isolated Kr atoms transition into a 1D gas and the single-atom contrast disappears in TEM. Nevertheless, complementary techniques of scanning TEM (STEM) imaging and electron energy loss spectroscopy (EELS) allowed us to track the movement of atoms within each nanotube through the mapping of chemical signatures.
“By focusing an electron beam to a diameter much smaller than the size of an atom, we can scan the entire nanotube and obtain spectra of the individual atoms trapped within it,” said Professor Quentin Lamasse, director of the EPSRC national research facility SuperSTEM. can be recorded.” Even if these atoms are moving. This gives a spectral map of the primary gas, confirming that the atoms are delocalized and fill all available space, just like in a normal gas. ”
Professor Paul Brown, director of the Nanoscale and Microscale Research Center (nmRC) at the University of Nottingham, said: “As far as we know, this is the first time that a chain of noble gas atoms has been directly imaged.”
The research team plans to use electron microscopy to image temperature-controlled phase transitions and chemical reactions in one-dimensional systems to uncover the secrets of such unusual states of matter.
For more information:
Atomic-scale time-resolved imaging of krypton dimers and chains and transitions to primary gas, ACS nano (2024). DOI: 10.1021/acsnano.3c07853
Quote: Scientists trap krypton atoms to form one-dimensional gas (January 22, 2024) https://phys.org/news/2024-01-scientists-krypton-atoms-Dimensional-gas.html Retrieved January 22, 2024 from
This document is subject to copyright. No part may be reproduced without written permission, except in fair dealing for personal study or research purposes. Content is provided for informational purposes only.